Dating back to an idea proposed in 2004, the past several years have witnessed the emergence of a new paradigm in alloy design, in which three or more elements are mixed in roughly equal proportions. Named multiprincipal element alloys (MPEAs), or often known as a subset of these alloys called high-entropy alloys, these materials blur the distinction between majority and minority populations of elements. This more perfect union of atomic partners that make up the collective material exhibit exciting properties that allow them to perform better than their traditional counterparts.
“Some of these materials exhibit exceptional combinations of strength, ductility, and damage tolerance,” writes a team of UC Santa Barbara researchers and their co-authors in a paper published today in the journal SCIENCE. “Refractory alloys [made from a group of nine metal elements on the periodic table that are highly resistant to heat and wear] are attractive candidates for use at extremely high temperatures associated with many technology applications.”
MPEAs motivated the development of refractory MPEAs, first made in 2010. But using multiple alloys almost infinitely increases the number of possible alloy “recipes.” The sheer number of combinations that can be achieved sets the stage for the use of advanced computational screening and machine learning to target the materials subsets having the most interesting and desirable properties.
“For these ap¬proaches to be successful, it is criti¬cal that the alloy design process is guided by an understanding of the origins of the specific properties that are desired,” writes Julie Cairney in a companion piece to the SCIENCE article. Cairney is a professor at the School of Aerospace, Mechanical and Mechatronic Engineering, at the University of Sydney, in Australia.
In the main SCIENCE article, the UCSB researchers, including materials professors Dan Gianola, Tresa Pollock, and Irene Beyerlein; UCSB postdoctoral researcher Fulin Wang, and colleagues at the University of Kentucky, the U.S. Naval Research Laboratory, and the U.S. Air Force Research Laboratory, suggest a way to enhance the ability to predict which alloys might have valuable properties.
Chief among such properties is an alloy’s ability to deform, i.e. be molded or bent, without cracking and to maintain its material integrity under the excessive loads and the high heat found in extreme environments, such as in airplane wings, rocket engines, and industrial turbines.
Wang, a postdoc in Gianola’s lab, explains what that means. “On the atomic level, a material deforms, or changes its shape, as a result of moving atoms,” he says.
The crystalline structures of metals are made up of stacked planes of atoms organized into a highly regular grid. When a metal deforms, atoms move, or slide, over each other on the grid. The line separating the regions where atoms have moved and where they have not is called a dislocation. The properties of dislocations, including how easily and where they can move, therefore become very important to the deformation behaviors of the material.
Despite the advantages of MPE alloys, progress in designing them has been slow. While traditional trial-and-error approaches are inefficient, from about 2017, more research efforts were devoted to developing theories to try to identify the underlying reason that a particular alloy had desirable properties. “But,” Wang says, “there is a lack of experimental evidence to inform some critical elements of the theory. When I started working on this project, my immediate question was, what’s special about the MPEAs compared to traditional alloys? Since we are interested in mechanical properties, we focus on the dislocations.”
In this study, the researchers used electron microscopy to investigate the configurations of dislocations and unveil the mechanistic origins that give rise to desirable properties in a model alloy. Combined with the atomistic simulations from the group of Irene Beyerlein, they showed that the random field of different elements unlocks multiple pathways for dislocation movements, features not available in conventional alloys.
“For conventional dislocations, the force to break atomic bonds at a dislocation is single valued because all the atoms are alike,” Beyerlein says. “For the MPE dislocation, this force cannot be deterministic. The structure of an MPE dislocation becomes redefined as it tries to move through randomly changing atomic environments.
“With our atomistic calculations, we took the approach of expecting the unexpected and probed not only the usual modes but additional higher modes of slip, typically neglected in the literature to date,” she adds. “We also performed thousands of calculations, which exposed just how widely varying this critical dislocation force can be and how favorable alternative higher modes of slip are.”
The study is part of a larger collaborative effort led by Tresa Pollock and funded by the Office of Naval Research, named MPE.edu, that also involves UCSB researchers Carlos Levi and Anton van der Ven, aimed at gaining fundamental insights regarding how best to explore the vast refractory alloy space.
“While compositionally complex alloys have long been of interest to us, progress in exploring the large compositional space has been slow,” says Pollock. “With the MPE project, we brought together a team that used emerging computational, machine learning, and experimental tools, which have enabled us to uncover new behaviors and rapidly explore new compositional domains. The very high melting points of the refractory materials of interest have made them notoriously difficult to fabricate and study in the past, but our new approaches, combined with the possibility of 3D printing, completely change the landscape.”
“This work is emblematic of the true power of combining experiments with simulation and theory,” Gianola says. “Many researchers pay lip service to this synergy, but this study could not have gone as far as it did without the constant back and forth between the experimental and simulation groups. The future looks very bright.”
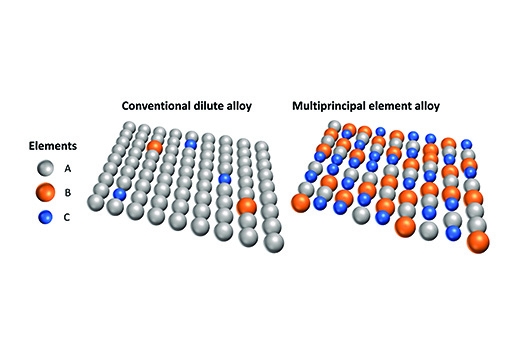
Blurring the lines between majority and minority populations of atomic species in a multiprincipal element alloy (right) leads to a rugged atomic landscape, opening up new pathways for defects to navigate.
